The TBB sample Collection
In 2023 we have collected around 2500 aliquots from 1700 patients bringing the total collection to 57500 aliquots from more than 18000 patients from different clinics that continue their important involvement in the biobank. The contribution per clinic can be found in the chart below, with the largest amount of samples deriving from the gynaecology clinic, followed by the clinics of visceral surgery, neurosurgery, thoracic surgery as well as urology.

Projects
TBB is actively contributing to research, with a rising number of prospective and retrospective projects as well as customized collections. The increasing number of projects is shown in the picture below.

TBB ACTIVITIES
TBB is an active core facility, putting a lot of emphasis on the development of new processes and methods to meet researcher's requirements.
In the last years, aside from providing the scientists with frozen and native material, we have increased the portfolio of fit for purpose collections, implying strong multi-institutional and cross-departmental collaboration. We work in close cooperation with the Histology Diagnostic of the Institute of Tissue Medicine and Pathology (ITMP) and the clinics, where the biobank tissue is already processed in the operating theatre allowing the maximum tissue quality necessary for particular studies. The quality of this tissues, in terms of RNA quality is excellent, with RNA integrity numbers (RIN) of 10 for most of the analysed samples.
The principal TBB on-going activities are summarised in the figure below.

Sample request
We are committed to simple processes for the researcher that save unnecessary bureaucratic time.
Feasibility studies do not need any official document, only a mail with the requirements. The information will be then retrieved and delivered as soon as possible.
Regarding the sample request, the only administrative work for the researcher is to fill in the application form with basic information about the project. Our general process is as follows.
Request form:
The official request starts upon receipt of the request form. It includes a short project outline, type and specifications of the requested material and basic information on ethics approval.
TBB order processing:
After the request, the project is presented to the scientific TBB committee to get the technical approval. Once the project is approved by the TBB, the information is sent to the corresponding Insel tissue sender department for clinical approval.
Once all acceptances are in place, the status of the patient's general consent is thoroughly checked. Only samples with signed general consent by the patient will be given.
Sample delivery:
The selected samples will undergo an exit quality control (H&E staining revision by an expert pathologist) to assure the coverage of the requirements. A quality report shall be issued.
At the time of the data and material transfer, a corresponding material transfer agreement document needs to be signed by the issuing and receiving parties. This defines the rights and obligations of both parties for the use of the data, materials and their derivatives.
The estimated timelines for each part of the process are displayed in the picture below.

Information for clinics
In addition to the simple sample acquisition and retrieval of frozen or native material, TBB has significantly expanded its portfolio in the direction of customised collections. An example of this, is the tissue cryopreservation for live cell cultures and the freezing of the sample in the operating theatre to reduce ischemia times to the minimum.
If you are interested in the services of the TBB for collecting tissue samples, please contact tissuebank.igmp@unibe.ch. The tissue will then be stored and managed in accordance with the legal regulations and according to the Swiss Biobanking Platform standards.
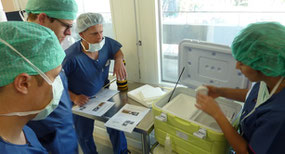
Personnel from the Clinic for Ear, Nose and Throat Diseases, Head and Neck Surgery, being trained to snap freeze the sample in the operating theatre.
This procedure, developed by TBB, leads to excellent quality samples due to cold ischemia times of less than 1 minute.
Information for patients
By signing the General Consent document provided by Inselspital at the time of surgery, the patients allow the usage of the left over material for diverse scientific projects, making a significant contribution to the prediction, prevention, diagnosis and treatment of human diseases that eventually will benefit other patients. The destination of the tissue (always left over material not needed for diagnosis) is a high-level research project that has been approved by an ethics committee and by the scientific committee of the TBB. The patient has always the right to change his or her mind as well and revoke the consent without the need of explaining the reasons. Such decision will not have any influence on his or her treatment.
We ensure strict compliance with patients' rights and transparency. TBB works accordingly with the Federal Act on Research involving Human Beings (HRA) and the Ordinance on Human Research with the Exception of Clinical Trials (HRO) and following the Swiss Biobanking Platform guidelines for the best biobanking practises.
References
As result of the increasing number of projects and efforts, the TBB has been referenced in numerous articles this year:
1. Adamczyk, A., Pastille, E., Kehrmann, J., Vu, V. P., Geffers, R., Wasmer, M. H., Kasper, S., Schuler, M., Lange, C. M., Muggli, B., Rau, T. T., Klein, D., Hansen, W., Krebs, P., Buer, J., & Westendorf, A. M. (2021). GPR15 facilitates recruitment of regulatory T cells to promote colorectal cancer. Cancer Research, 81(11), 2970–2982. doi: 10.1158/0008-5472.CAN-20-2133
2. April-Monn, S. L., Andreasi, V., Lena, M. S., Sadowski, M. C., Kim-Fuchs, C., Buri, M. C., Ketkar, A., Maire, R., di Domenico, A., Schrader, J., Muffatti, F., Doglioni, C., Partelli, S., Falconi, M., Perren, A., & Marinoni, I. (2021). Ezh2 inhibition as new epigenetic treatment option for pancreatic neuroendocrine neoplasms (Pannens). Cancers, 13(19). doi: 10.3390/cancers13195014
3. Depeursinge, A., Racoceanu, D., Iavindrasana, J., Cohen, G., Platon, A., Poletti, P.-A., & Muller, H. (2010). Fusing Visual and Clinical Information for Lung Tissue Classification in HRCT Data. Artificial Intelligence in Medicine, ARTMED1118. doi: 10.1016/j
4. di Domenico, A., Pipinikas, C. P., Maire, R. S., Bräutigam, K., Simillion, C., Dettmer, M. S., Vassella, E., Thirlwell, C., Perren, A., & Marinoni, I. (2020). Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Communications Biology, 3(1). doi: 10.1038/s42003-020-01479-y
5. Ercolano, G., Gomez-Cadena, A., Dumauthioz, N., Vanoni, G., Kreutzfeldt, M., Wyss, T., Michalik, L., Loyon, R., Ianaro, A., Ho, P. C., Borg, C., Kopf, M., Merkler, D., Krebs, P., Romero, P., Trabanelli, S., & Jandus, C. (2021). PPARɣ drives IL-33-dependent ILC2 pro-tumoral functions. Nature Communications, 12(1). doi: 10.1038/s41467-021-22764-2
6. Gao, Y., Zens, P., Su, M., Gemperli, C. A., Yang, H., Deng, H., Yang, Z., Xu, D., Hall, S. R. R., Berezowska, S., Dorn, P., Peng, R. W., Schmid, R. A., Wang, W., & Marti, T. M. (2021). Chemotherapy-induced CDA expression renders resistant non-small cell lung cancer cells sensitive to 5′-deoxy-5-fluorocytidine (5′-DFCR). Journal of Experimental and Clinical Cancer Research, 40(1). doi: 10.1186/s13046-021-01938-2
7. Karkampouna, S., la Manna, F., Benjak, A., Kiener, M., de Menna, M., Zoni, E., Grosjean, J., Klima, I., Garofoli, A., Bolis, M., Vallerga, A., Theurillat, J. P., de Filippo, M. R., Genitsch, V., Keller, D., Booij, T. H., Stirnimann, C. U., Eng, K., Sboner, A., … Kruithof-de Julio, M. (2021). Patient-derived xenografts and organoids model therapy response in prostate cancer. Nature Communications, 12(1). doi: 10.1038/s41467-021-21300-6
8. Leimkühler, N. B., Gleitz, H. F. E., Ronghui, L., Snoeren, I. A. M., Fuchs, S. N. R., Nagai, J. S., Banjanin, B., Lam, K. H., Vogl, T., Kuppe, C., Stalmann, U. S. A., Büsche, G., Kreipe, H., Gütgemann, I., Krebs, P., Banz, Y., Boor, P., Tai, E. W. Y., Brümmendorf, T. H., … Schneider, R. K. (2021). Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell, 28(4), 637-652.e8. doi: 10.1016/j.stem.2020.11.004
9. Losmanova, T., Zens, P., Scherz, A., Schmid, R. A., Tschan, M. P., & Berezowska, S. (2021). Chaperone-mediated autophagy markers lamp2a and hspa8 in advanced non-small cell lung cancer after neoadjuvant therapy. Cells, 10(10). doi: 10.3390/cells10102731
10. Vassella, E., Kashani, E., Zens, P., Kündig, A., Fung, C., Scherz, A., Herrmann, E., Ermis, E., Schmid, R. A., & Berezowska, S. (2021). Mutational profiles of primary pulmonary adenocarcinoma and paired brain metastases disclose the importance of KRAS mutations. European Journal of Cancer, 159, 227–236. doi: 10.1016/j.ejca.2021.10.006
11. V’kovski, P., Gultom, M., Kelly, J. N., Steiner, S., Russeil, J., Mangeat, B., Cora, E., Pezoldt, J., Holwerda, M., Kratzel, A., Laloli, L., Wider, M., Portmann, J., Tran, T., Ebert, N., Stalder, H., Hartmann, R., Gardeux, V., Alpern, D., … Dijkman, R. (2021). Disparate temperature-dependent virus–host dynamics for SARS-CoV-2 and SARS-CoV in the human respiratory epithelium. PLoS Biology, 19(3). doi: 10.1371/journal.pbio.3001158
12. Yang, Z., Liang, S. Q., Yang, H., Xu, D., Bruggmann, R., Gao, Y., Deng, H., Berezowska, S., Hall, S. R. R., Marti, T. M., Kocher, G. J., Zhou, Q., Schmid, R. A., & Peng, R. W. (2021). CRISPR-mediated kinome editing prioritizes a synergistic combination therapy for FGFR1-amplified lung cancer. Cancer Research, 81(11), 3121–3133. doi: 10.1158/0008-5472.CAN-20-2276
13. Zens, P., Bello, C., Scherz, A., Koenigsdorf, J., Pöllinger, A., Schmid, R. A., Ochsenbein, A., Neppl, C., Langer, R., & Berezowska, S. (2021). A prognostic score for non-small cell lung cancer resected after neoadjuvant therapy in comparison with the tumor-node-metastases classification and major pathological response. Modern Pathology, 34(7), 1333–1344. doi: 10.1038/s41379-021-00777-y
